Virus Update
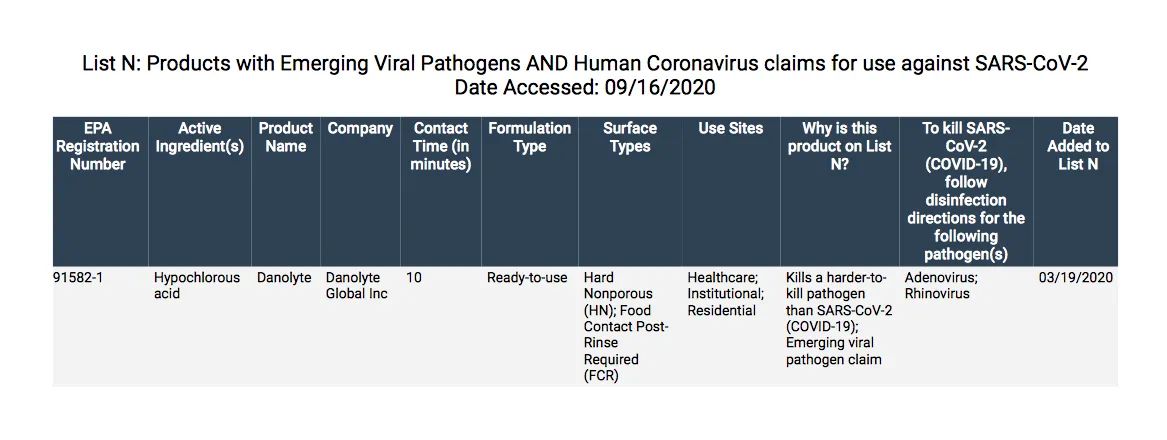
Danolyte has demonstrated effectiveness against viruses similar to SARS-CoV-2 on hard porous and/or non-porous surfaces. Therefore, Danolyte can be used against SARS-CoV-2 when used in accordance with the directions for use against Adenovirus Type 1, on hard, porous/non-porous surfaces. Refer to the CDC website at www.cdc.gov/outbreaks or OEI website at https://www.oie for additional information.
HOME
OFFICE
HEALTHCARE
EDUCATION
INDUSTRIAL
Danolyte Label Copy
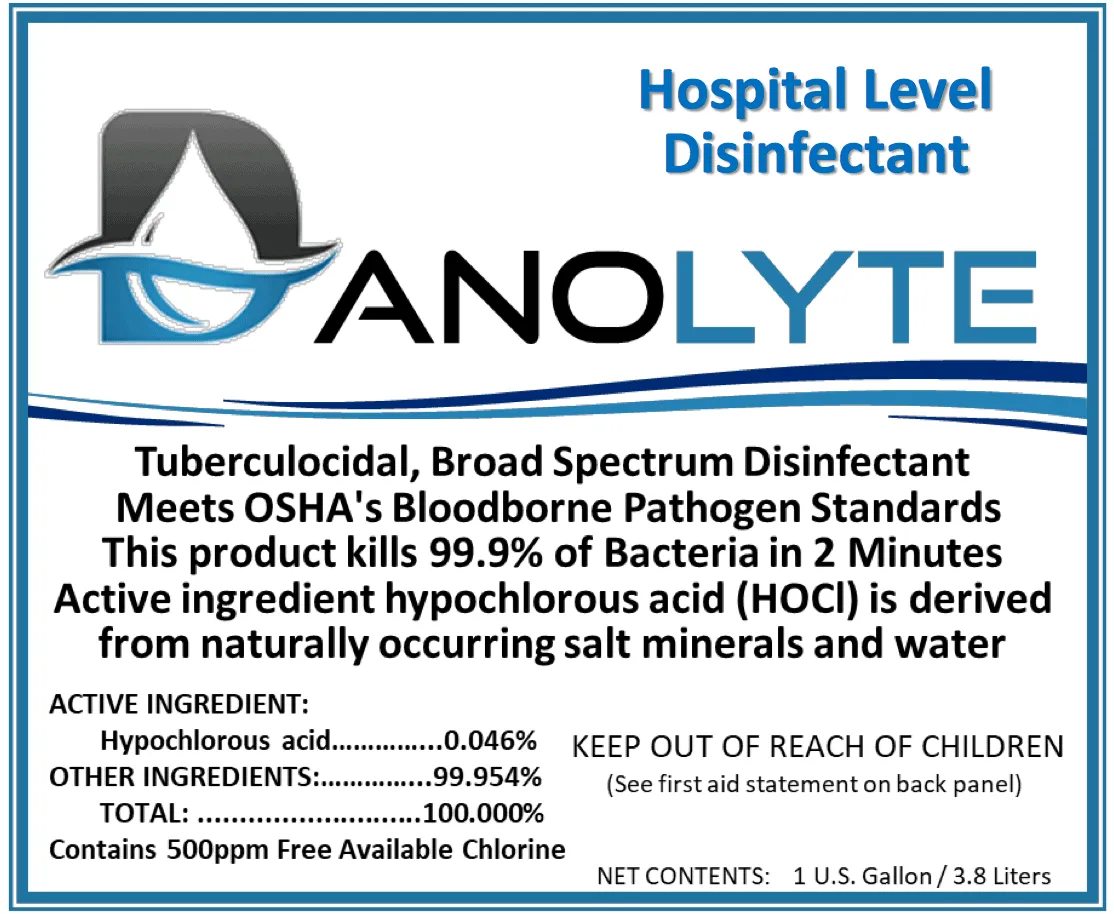
Hospital-Level Disinfectant
Danolyte
Tuberculocidal, Broad Spectrum Disinfectant
Meets OSHA’s Bloodborne Pathogen Standards
This Product kills 99.9% of Bacteria in 2 minutes
From naturally occurring salt minerals and water
Active Ingredient:
Hypochlorous acid……0,046%
Other ingredients: 99.954%
Total: …………………100.000%
Contains 500ppm free available chlorine
Keep out of reach of children
(see first aid statement on back panel)
Net Contents: 1 U. S. Gallon 3.8 Liters
DIRECTIONS FOR USE It is a violation of Federal law to use this product in a manner inconsistent with its labeling. DISINFECTION APPLICATIONS Hard Non-Porous Surface Disinfection: To Clean, Disinfect and Deodorize Hard, Non-Porous Surfaces: For heavily soiled areas, a preliminary cleaning is required. Apply, wipe, spray, or dip Danolyte® at 500 ppm FAC to hard, non-porous surfaces with a cloth, wipe, mop or sponge. Treated surfaces must remain wet for 10 minutes. Allow surfaces to air dry. This product is not to be used as a terminal sterilant/high level disinfectant on any surface or instrument that (1) is introduced directly into the human body, or (2) contacts intact mucous membranes but which does not ordinarily penetrate the blood barrier or otherwise enter normally sterile areas of the body. This product may be used to pre-clean or decontaminate critical or semi-critical devices prior to sterilization or high-level disinfection.
Special Instructions for Cleaning Prior to Disinfection against Clostridium difficile endospores:
Personal Protection: Wear appropriate barrier protection such as gloves, gowns, masks, or eye covering.
Cleaning Procedure: Fecal matter/waste must be thoroughly cleaned from surfaces/objects before disinfection by application with clean cloth, mop, and/or sponge saturated with product intended for disinfection. Cleaning should include vigorous wiping and scrubbing, until visible soil is removed. Special attention is needed for high-touch surfaces. Surfaces in patient rooms are to be cleaned in an appropriate manner, such as from right to left or left to right, on horizontal surfaces, and top to bottom, on vertical surfaces, to minimize spreading of the spores. Restrooms are to be cleaned last. Do not reuse soiled cloths.
Infectious Materials Disposal: Cleaning materials used that may contain feces/wastes should be disposed of immediately in accordance with local regulations for infectious materials disposal. Killing Clostridium difficile: Clean hard, non-porous surfaces by removing gross filth, loose dirt, debris, blood/bodily fluids, etc. Apply this product and let stand for 10 minutes.
Special Instructions for Using this product to Clean and Decontaminate against HIV on Surfaces/Objects Soiled with Blood/Body Fluids: This product kills HIV-1 on pre-cleaned environmental surfaces/objects previously soiled with blood/body fluids in health care settings (e.g. hospitals) or other settings in which there is an expected likelihood of soiling of inanimate surfaces/objects with blood or body fluids, and in which the surfaces/objects likely to be soiled with blood or body fluids can be associated with the potential for transmission of Human Immunodeficiency Virus Type 1 (HIV-1) associated with AIDS. Personal Protection: When handling items soiled with blood or body fluids, use appropriate barrier protection such as disposable latex gloves, gowns, masks, and eye coverings.
Cleaning Procedure: Blood and other body fluids must be thoroughly cleaned from surfaces and other objects before applying this product.
Contact Time: Apply to area to be treated. Let stand for 10 minutes. Cleaning materials used that may contain feces/wastes should be disposed of in accordance with local regulations for infectious materials disposal.
Disposal of Infectious Material: Blood and other body fluids must be autoclaved and disposed of according to local regulations for infectious waste disposal.
Organisms for Disinfection Applications:
(Contact Time: 10 minutes, unless otherwise noted )
BACTERIA:
Clostridium difficile – spore (C. Diff) (ATCC 43598); Escherichia coli (ATCC 11229); Klebsiella pneumonia New Delhi Metallo-Beta Lactamase (NDM-1) Carbapenem Resistant, CDC (10002); Listeria monocytogenes (ATCC 7644); Methicillin Resistant Staphylococcus aureus (MRSA) (ATCC 33591); Pseudomonas aeruginosa (ATCC 15442); Salmonella enterica (ATCC 10708); Staphylococcus aureus (ATCC 6538); Vancomycin Resistant Enterococcus faecalis (VRE) (ATCC 51229); Bordetella bronchiseptica (ATCC 10580)
MYCOBACTERIUM:
Mycobacterium bovis, BCG (Tuberculosis or TB) VIRUSES NON-ENVELOPED: Adenovirus (1 or Type 1) (Strain 71) (ATCC VR-1); Norovirus or Norwalk Virus (as Feline Calicivirus)(Strain F-9)(ATCC VR-782); Rhinovirus (16 or Type 16) (Strain 11757) (ATCC VR-283); Rotavirus (A or Group A) (Strain WA) (ATCC VR-2018); VIRUSES ENVELOPED: Hepatitis C Virus (2 minutes) (as bovine diarrhea virus) (HCV) (Strain ADL) (ATCC VR-1422); Human Immunodeficiency Virus Type 1 (HIV-1), strain IIIB (clade B) ZeptoMetrix; Influenza A (H1N1) Virus (2 minutes) (Strain A/Virginia/ATCC1/2009) (ATCC VR-1736) (flu virus); Respiratory Syncytial Virus (RSV) (Strain A-2) (ATCC VR-1540); Swine Flu Virus (H1N1) A/Swine/1976/31 (ATCC VR-99); Canine distemper (ATCC VR-1587) [(Strain Snyder Hill)];
PARVOVIRUS NON-ENVELOPED:
Canine parvovirus (ATCC VR-2016) [(Strain Cornell)]
YEAST:
Candida albicans (ATCC 10231);
Danolyte® is an activated aqueous solution of hypochlorous acid produced by passing weak salt brine through an electrolytic cell using Electro-Chemical Activation (ECA) technology to temporarily change the properties of dilute salt water into a powerful oxidizing agent exhibiting antimicrobial properties. Danolyte® is produced at a near neutral 6.5 pH where the predominant antimicrobial agent is hypochlorous acid, an efficient and efficacious specie of chlorine. Hypochlorous acid kills bacteria. When produced, Danolyte® (an anolyte solution), contains a minimum of 500 ppm free available chlorine (FAC).
Danolyte® cleans and disinfects: hospitals, medical clinics, ambulances, emergency rooms, dentist’s offices, home health care settings, funeral homes, correctional facilities, dormitories, colleges, schools, day care centers, churches, gymnasiums, locker rooms, hotels, cruise ships, airplanes, trains, yachts, campers, food processing plants, restaurants, bars, grocery stores, veterinary facilities, kennels, pet shops, public facilities, and homes.
SANITIZING APPLICATIONS
This product is an effective multi-purpose sanitizer. This product is acceptable as a sanitizer for all hard non-porous surfaces in and around food processing areas. Hard, Non-Porous Non-Food Contact Surfaces:
Danolyte Label Copy
Sanitize Hard, Non-Porous Non-Food Contact Surfaces: For heavily soiled areas, a preliminary cleaning is required. Dilute 1:1.5 with water to prepare a 200 ppm FAC solution. May use chlorine test strips as an option to determine exact available chlorine concentration. Apply sanitizing solution with cloth, mop, sponge, spray or immersion. Treated surfaces must remain wet for 2 minutes. Allow surfaces to air dry. Danolyte® is an effective cleaner/sanitizer against bacteria such as Staphylococcus aureus (Staph) and Enterobacter aerogenes. This product kills 99.9% of bacteria with a 5% organic soil load in two minutes.
To Deodorize: Spray on surfaces as needed.
Hard, Non-Porous Food Contact Surfaces:
Sanitize Hard, Non-Porous Food Contact Surfaces: Dilute 1:1.5 with water to prepare a 200 ppm FAC solution. May use chlorine test strips as an option to adjust to desired chlorine level. Wash, wipe, or rinse items with detergent and water, then apply sanitizing solution with cloth, mop, sponge, spray or immersion. Let stand 1 minute and wipe dry with clean towel or allow to air dry. No rinsing required. For use on food contact surfaces such as [exterior surfaces of coolers, refrigerators, freezers, microwave ovens, ovens and stove tops which should be allowed to come to room temperature before sanitization, ]stainless steel utensils, plastic and nonporous cutting boards and chopping blocks, dishes, glassware, pots and pans, eating and cooking utensils, sinks, counter tops, tables, racks, carts, shelves, appliances, conveyor belts. This product is an effective sanitizer against Staphylococcus aureus (Staph) and Salmonella enterica. This product is an effective sanitizer against Staphylococcus aureus (Staph) and Salmonella enterica (Salmonella).
To Use as a Glove Dip or Boot Wash: Dilute this product 1:1.5 with water to prepare a 200 ppm FAC solution. May use chlorine test strips as an option to adjust to desired chlorine level. This product meets AOAC Available Chlorine in Disinfectants chlorine equivalency against Salmonella enterica (ATCC 6539) and Staphylococcus aureus (ATCC 6538). This product meets the requirements of 2-301.16 Hand Antiseptics section of the U.S. PUBLIC
HEALTH SERVICE FDA FOOD CODE.
ALLERGEN DESTRUCTION APPLICATIONS:
To Destroy Specified Allergens: Dilute 1:4 to1:1.5 with water to prepare a 100-200 ppm FAC sanitizing solution. As an option, use chlorine test strips to adjust to desired chlorine level. Apply sanitizing solution with paper towel, cloth, mop, sponge, spray or immersion. Treated surfaces must remain wet for 2 minutes.
Allow surfaces to air dry. This product breaks down and destroys allergens: dust mite matter, dust mite debris, cockroach matter, cockroach debris, pet dander, dog dander, cat dander and pollen particles. Use often as desired.
Cut Flowers or Plants: For longevity of cut flowers or plants mix 1-2 ounces [(1/8 – 1/4 cup)] of Danolyte® per quart of water to make a 15-30 ppm FAC solution for use in flower vase or buckets to retard the growth of non-public health bacteria. Change solution if it gets murky or hazy. Spray diluted solution on plants or flowers to control bacteria growth.
Sanitize Water Sensitive Electronic Equipment, Surfaces: Completely power off electrical equipment prior to treatment. Pre-clean soils from external surfaces to be sanitized with a clean paper towel, cloth, microfiber, or sponge, which may be dry or slightly wetted with this product. Dilute this product 1:1.5 with water to prepare a 200 ppm FAC solution. May use chlorine test strips as an option to adjust to desired chlorine level. Carefully apply sanitizing solution using a cloth or spray device so that only enough solution is applied to keep the surface thoroughly wet for 2 minutes. Avoid over soaking and prevent pooled or puddled areas. Treated surfaces must remain wet for 2 minutes. Reapply as necessary to keep wet for 2 minutes. Do not rinse. Allow surfaces to air dry. If hazy film or streaks appear after 2 minutes, wipe clean with a dry or slightly damp clean paper towel microfiber cloth. Do not restore power to electronic equipment until thoroughly dry.
ORGANISMS FOR SANITIZING APPLICATIONS
Non-Food Contact Surface Bacteria - Contact Time: 2 minutes
Enterobacter aerogenes (ATCC 13408); Staphylococcus aureus (ATCC 6538)
Food-Contact Surface Bacteria - Contact Time: 60 seconds
Salmonella enterica (ATCC 6539); Staphylococcus aureus (ATCC 6538)
STORAGE AND DISPOSAL
Do not contaminate water, food or feed by storage or disposal.
Storage: Store in a closed dark plastic container away from direct sunlight. Store in a cool dry area. Product may be disposed in a sanitary sewer.
Pesticide Disposal: Wastes resulting from the use of this product must be disposed of on-site or at an approved waste disposal facility.
Container Disposal: Refillable container. Refill this container with same product only. Do not reuse this container for any other purpose. Cleaning before refilling is the responsibility of the refiller. Cleaning the container before final disposal is the responsibility of the person disposing the container. To clean the container before final disposal, empty the remaining contents from this container into application equipment or mix tank. Fill the container about 10 percent full with water. Agitate vigorously or recirculate water with the pump for two minutes.
Repeat this rinsing procedure two more times. Then offer for recycling if available, or puncture and dispose of in a sanitary landfill, or by incineration, or by other procedures allowed by state and local authorities.
EPA Reg. No. 91582-1 EPA Est. No. 91582-KS-1 Date Produced:
Danolyte® must be used for disinfection applications within 30 days after being produced OR product must be diluted and, as an option, may be tested with chlorine test kit or chlorine test strips to adjust to desired chlorine level for sanitizing, deodorizing, and cleaning applications.
Environmental Commitment
This product rapidly breaks down entirely to salt water. Not harmful to septic and sewer systems. This bottle is coded for recyclers. Check to see if recycling facilities accept colored HDPE. Contains no phosphorous, VOCs, alcohol or phenols. Low Odor. Fresh and clean scent.
This product meets AOAC efficacy testing standards for hospital disinfection. Meets requirements of OSHA’s Bloodborne Pathogen Guidelines. Do not use on steel, aluminum, silver, or chipped enamel. Prolonged contact with metal may cause pitting or discoloration. Test in an inconspicuous place for color washout or contact incompatibility.
FIRST AID
Call a poison control center or doctor for treatment advice. Have the product container or label with you when calling a poison control center or doctor, or going for treatment. You may also contact the National Pesticide Information Center (NPIC) 1-800-858-7378 for emergency medical treatment information.
What We Do
We design, manufacture and market generators which produce a new generation of disinfectant. Danolyte Global’s machines produce a powerful antimicrobial fluid for use across a broad spectrum of applications, yet gentle enough for use on baby toys. Our unique technology offers innovative disinfection, sanitizing and deodorizing solutions for many industries including water treatment, agriculture, oil and gas, healthcare, education, and food service/processing.
Our History
Danolyte Global, Inc. acquired the assets of the Italian company Duedi, Sri in January 2015, which included proprietary technologies and intellectual property concerning the manufacture and application of anolyte generating equipment. Recognizing the high demand prospects for the solutions offered by this technology and the challenges of meeting that demand, the company undertook design improvements to allow for highly scalable production. This redesign included full automation of the equipment and upgrades to critical components to make our generators the most efficient and durable systems available.